
This is one of the characteristics of the carbon atom which is very important for you to know. The covalent bond on the carbon atom is what produces the electron configuration of carbon with a total of 6. Thus, the bonds between the carbon atoms will form a covalent bond.

You need to remember that the carbon atom is a nonmetal element in group IVA. Read also: History of discovery of electrons, negatively charged subatomic particles Carbon Electron Configuration is an element of the periodic table and its atomic number is 6. These configurations are represented graphically in Figure 1, with the electrons, shown as white dots, occupying the orbitals, whose geometrical forms are drawn. Quaternary carbon atom: The type that binds directly to four other carbon atoms.Tertiary carbon atom: The type that directly bonds to three other carbon atoms. Electron configuration of Carbon: In atomic physics and quantum chemistry, electron configuration is the distribution of the electrons of an atom or molecule into atomic or molecular orbitals.
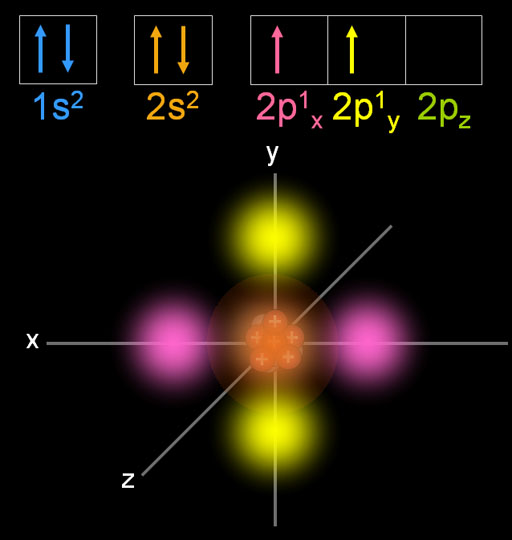
Secondary Carbon Atom: A type of atom directly bonded to two other carbon atoms.A distinctive feature of the next atom is. The carbon atom has a total of 4 valence electrons which can be covalently bonded to similar atoms or other atoms. This will determine the ability of the carbon atoms to form long C chains. Primary Carbon Atom: A type of carbon atom that only directly or bonded one atom to another. For its covalent bonds, the carbon atom has a unique characteristic with atomic number 6 electron configuration.Possible types of atomic positions are divided into 4, namely: So, the more possibilities, the more types of compounds that can be formed by carbon atoms.

From the Bohr model of Carbon, we know, that 2 electrons are in the K-shell and 4 electrons are in the L-shell. The carbon atom itself can bond with other atoms so that its potential position in the bonding chain will depend on the number.Įvery possibility that exists can produce one type of compound. The electron configuration of Carbon Electron configuration is the distribution of electrons of an atom or molecule in atomic or molecule orbitals Carbon has an atomic number of 6 and it contains a total number of 6 electrons. First you know the characteristics of the carbon atom to determine the number of electron configurations of carbon.


 0 kommentar(er)
0 kommentar(er)
